X-chromosome inactivation is a fascinating biological process that plays a crucial role in ensuring balance between male and female genetic expression. In females, who possess two X chromosomes, one of these is inactivated to prevent a doubling of X-linked gene dosage, a phenomenon that can lead to significant genetic disorders such as Fragile X Syndrome and Rett Syndrome. This intricate mechanism involves the action of Xist RNA, which orchestrates chromosomal silencing, allowing cells to effectively manage their genetic resources. By understanding X-chromosome inactivation, researchers have the potential to unlock new therapies aimed at treating these and other X-linked conditions. Recent studies have revealed surprising insights into this process, offering hope for future advancements in gene therapies.
The process often referred to as X chromosomal silencing is essential for equalizing gene expression in females, who have a unique genetic setup compared to males. With two X chromosomes, females face the challenge of managing these extra genes, which can lead to complications associated with various genetic disorders, including those resulting from mutations in the X chromosome. Xist RNA plays a pivotal role in this phenomenon, guiding the inactivation process and providing insights into how we can address disorders like Fragile X Syndrome and Rett Syndrome. Understanding the mechanics behind X chromosomal regulation opens the door to innovative treatment approaches, potentially allowing us to reactivate silenced genes and alleviate symptoms of these conditions. This critical research is setting the stage for breakthroughs in the field of genetics and therapeutic developments.
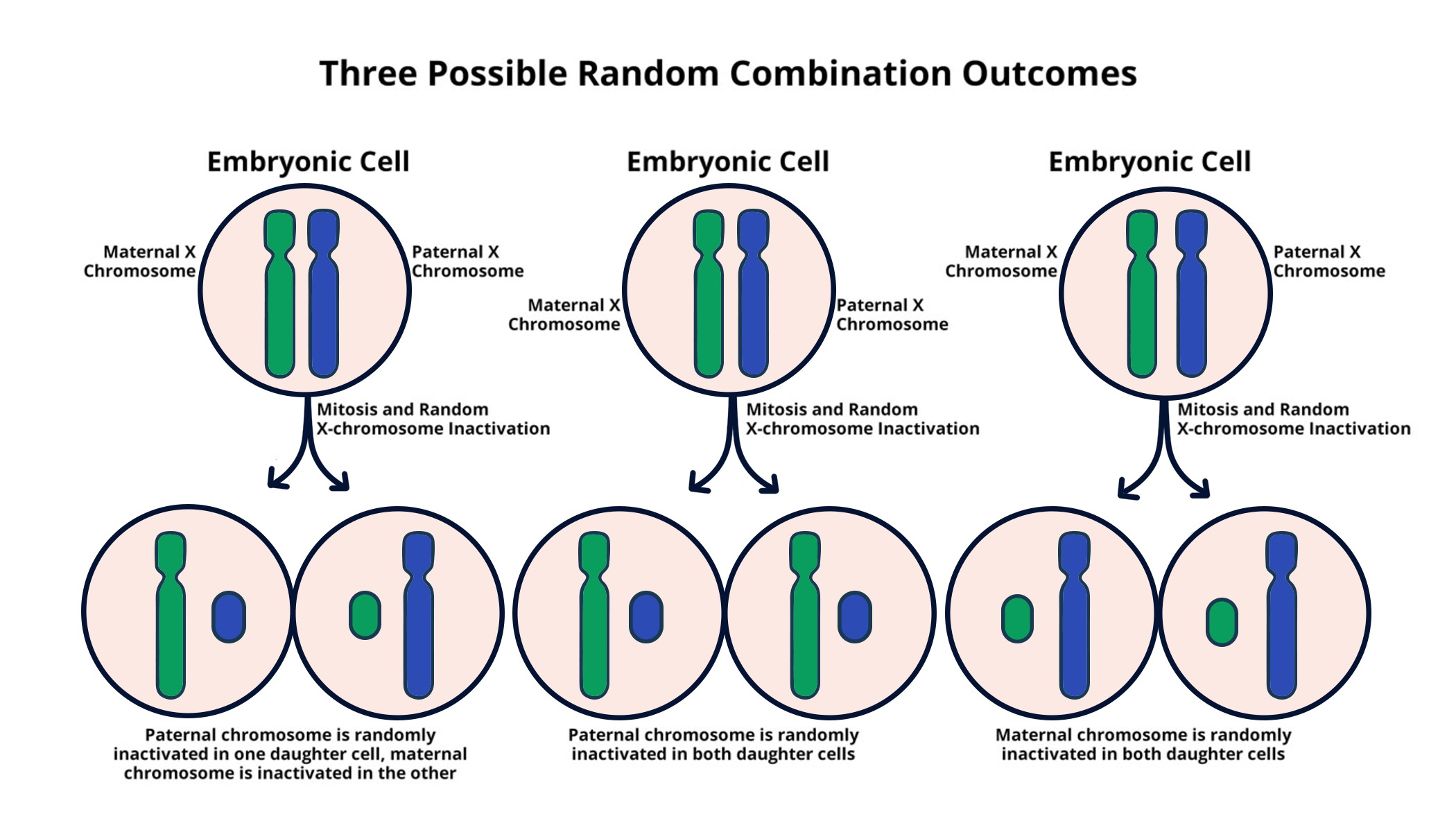
Understanding X-Chromosome Inactivation
X-chromosome inactivation (XCI) is a critical biological process unique to females, where one of the two X chromosomes is inactivated to prevent an overdose of X-linked gene expression. This intricate mechanism ensures dosage compensation between sexes, allowing females to express genes from just one X chromosome while males rely solely on their single X chromosome. The process of XCI is facilitated by a specialized RNA molecule called Xist, which plays a pivotal role in silencing the inactivated X chromosome. This discovery has not only reshaped our understanding of genetic regulation but also opened pathways to explore potential therapies for conditions associated with X chromosomal mutations.
Research shows that Xist engages in a complex interaction with a viscous substance that surrounds chromosomes—referred to metaphorically as ‘Jell-O.’ This substance helps chromosomes maintain their structure, preventing them from entangling. When Xist coats the chromosome, it alters the properties of this ‘Jell-O,’ facilitating the silencing process. Understanding how XCI operates at a molecular level not only illuminates basic biological principles but also holds promise for therapeutic interventions targeting genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
The Role of Xist RNA in Gene Regulation
Xist RNA is central to the mechanism of X-chromosome inactivation, acting as a regulator that initiates the silencing of one X chromosome in female mammals. When Xist is expressed, it coats the entire X chromosome, effectively changing its physical state and making it inaccessible for transcription. This is achieved through its interactions with chromatin and the surrounding ‘Jell-O’ substance, which collectively contribute to the formation of heterochromatin—a transcriptionally inactive form of chromatin. The detailed mechanisms of how Xist orchestrates this process are still under investigation but represent a keystone in understanding X-linked genetic disorders.
The implications of Xist RNA extend beyond XCI; its study provides a framework for exploring therapies for genetic disorders such as Fragile X Syndrome. In this condition, mutations on the X chromosome are responsible for significant intellectual disabilities, and recent advancements in the manipulation of gene expression via Xist could lead to groundbreaking treatments. By unsilencing mutated genes, researchers aim to restore functionality to affected cells, potentially offering hope not only to females with X-linked conditions but also to males who may express similar disorders due to X-linked mutations.
Implications for Genetic Disorders: Fragile X and Rett Syndromes
The research spearheaded by Jeannie T. Lee’s lab suggests promising new therapies for genetic disorders rooted in X-chromosome abnormalities, particularly Fragile X Syndrome and Rett Syndrome. Fragile X Syndrome is characterized by a permutation in the FMR1 gene on the X chromosome, leading to developmental delays and cognitive challenges. By harnessing the knowledge gained from Xist RNA’s function, scientists hope to develop methods to unsilence and express the healthy copy of the FMR1 gene in affected individuals. This could revolutionize treatment for those afflicted with this debilitating condition.
Similarly, Rett Syndrome, a neurodevelopmental disorder predominantly affecting females, also stems from mutations in the MECP2 gene on the X chromosome. The application of techniques derived from understanding XCI could provide crucial insights into reinstating the normal function of these vital genes. As research progresses, the therapeutic potential of targeting these X-linked genetic disorders may offer much-needed solutions and improve the quality of life for thousands, guiding us closer to effective interventions and a deeper understanding of X-linked gene function.
Chromosomal Silencing and Its Therapeutic Potential
Chromosomal silencing through X-chromosome inactivation is a fascinating area of genetics that holds significant promise for therapeutic applications. The ability to selectively silence and unsilence genes on the X chromosome can provide a targeted approach to treatment for various genetic disorders, including Fragile X Syndrome and Rett Syndrome. Researchers are now looking into using Xist RNA as a tool not only for understanding gene expression regulation but also for developing gene therapy approaches that could reactivate silenced genes and render inactive mutations functional again.
The therapeutic potential of these discoveries is vast. For instance, experimental strategies designed to manipulate Xist RNA offer hope for re-establishing gene function in patients with X-linked disorders. By learning how to control the inactivation and reactivation of genes on the X chromosome, scientists are unlocking ways to cure genetic disorders without affecting the expression of healthy alleles. This fine-tuning could lead to personalized medical strategies as researchers continue uncovering the nuanced dynamics of chromosomal silencing.
Bubbles and the Jell-O Analogy in Genetic Research
The unique property of the ‘Jell-O-like’ substance surrounding chromosomes plays a crucial role in their organization and function. This gelatinous coating is vital for protecting chromosomes and preventing them from becoming entangled, which is especially critical during cell division. The analogy to Jell-O helps illustrate the fluidity required for proper gene regulation, particularly concerning the X chromosomal inactivation process where Xist RNA interacts with this protective layer to induce silencing. Understanding this physical environment offers insights into the molecular dynamics at play and how disruptions might lead to genetic disorders.
Moreover, the intricate balance within this ‘Jell-O’ environment is crucial for maintaining both normal cellular function and gene expression. The study of the biophysical properties of this chromosomal coating enables researchers to identify potential new therapeutic targets that could correct abnormalities in gene expression linked to X-linked diseases. By revitalizing areas of genetic research focused on the structural intricacies of chromosomal configurations, scientists can better understand the pathways that lead to disorders such as Fragile X and Rett Syndromes.
The Evolution of Research on X-Chromosome Inactivation
The journey into understanding X-chromosome inactivation has undergone significant evolution since its inception. Initial studies focused solely on the fundamental mechanisms, wherein researchers sought to elucidate how females manage to balance gene expression between their two X chromosomes. Jeannie T. Lee’s groundbreaking work has streamlined this exploration, leading to a clearer understanding of the role of Xist RNA and the resultant cellular transformations necessary for silencing one of the X chromosomes. Over the decades, the focus has shifted toward potential clinical applications, marking a pivotal transition from basic scientific inquiry to applicable medical interventions.
With advancements in genetic research technologies, scientists are now equipped to unravel the mysteries of XCI with greater precision. The shift towards therapeutic exploration underscores the application of fundamental findings about X inactivation in addressing real-world health challenges. This progression highlights the critical nature of continual research support, like that from the National Institutes of Health, that allows for the translation of basic science into clinical innovations which might one day alter the course of treatment for individuals suffering from X-linked genetic disorders.
Current Perspectives on Gene Therapy and XCI
As our understanding of X-chromosome inactivation deepens, the possibilities for gene therapy seem to expand. The exploration of unsilencing techniques through Xist RNA manipulation gives hope that even those with profound genetic disorders caused by X-linked mutations can access effective treatments. The current perspectives within genetic research prioritize the exploration of these methodologies not only to treat existing conditions but also as preventive measures for future generations, spotlighting the need for more expansive clinical trials to assess safety and efficacy.
The burgeoning field of gene therapy tied to X-linked disorders emphasizes the significance of collaboration among researchers, clinical practitioners, and regulatory bodies. It is imperative to navigate the landscape of genetic therapies judiciously, ensuring that new approaches provide safe, reliable solutions while paving the way for further breakthroughs in the domain of genetic medicine. As investigations into XCI continue to reveal intricate details of chromosomal behavior, the dream of curing conditions like Fragile X and Rett Syndromes grows increasingly feasible.
Future Directions in X-Linked Genetic Research
Future directions in research surrounding X-linked genetic disorders are increasingly focusing on the translational aspects of findings from basic science. With tools and methodologies becoming more refined, researchers are investigating innovative strategies to manipulate gene expression directly via paths uncovered by studying chromosomal frameworks, particularly through the role of Xist RNA and its impact on X-chromosome inactivation. The potential for targeted gene therapy applications is enormous, promising advancements that could significantly alter the landscape of treatment options for Fragile X and Rett Syndromes.
The hope is that future studies will continue to refine our capabilities to unsilence mutated genes, creating tailored solutions for individuals affected by these disorders. As genetic research continues to evolve, it is crucial that scientists remain engaged with ethical considerations, particularly regarding genetic manipulation and the implications of unsilencing certain genes. With increased dialogue among stakeholders in genetics, medicine, and ethics, the pursuit of innovative therapies for X-linked disorders could usher in a new era of medical interventions.
Frequently Asked Questions
What is X-chromosome inactivation and why is it important?
X-chromosome inactivation is a biological process that occurs in female mammals where one of the two X chromosomes is randomly silenced. This is crucial for balancing gene dosage between males, who have one X chromosome, and females, who have two. Understanding X-chromosome inactivation is vital for addressing various genetic disorders linked to the X chromosome, such as Fragile X Syndrome and Rett Syndrome.
How does Xist RNA play a role in X-chromosome inactivation?
Xist RNA is essential for X-chromosome inactivation as it coats the X chromosome and initiates the silencing process. By modifying the biophysical properties of the surrounding chromosomal material, sometimes referred to as ‘Jell-O’, Xist allows other important molecules to access and modify the X chromosome, ultimately rendering it inactive.
What genetic disorders are associated with issues related to X-chromosome inactivation?
Genetic disorders like Fragile X Syndrome and Rett Syndrome are associated with mutations on the X chromosome. These disorders can arise when the mechanism of X-chromosome inactivation fails, leading to improper gene expression and resulting health challenges.
Can understanding X-chromosome inactivation lead to treatments for genetic disorders?
Yes, knowledge about X-chromosome inactivation can guide the development of therapies aimed at ‘unsilencing’ mutated genes on the inactive X chromosome. This approach holds promise for treating conditions such as Fragile X Syndrome and Rett Syndrome by restoring function to previously silenced genes.
What is the significance of chromosomal silencing in genetic research?
Chromosomal silencing, particularly regarding X-chromosome inactivation, is significant in genetic research as it provides insights into gene regulation. By studying this phenomenon, researchers can explore potential therapies for genetic disorders, improving our understanding of gene function and the intricate balance required for normal development.
How could freeing inactivated X chromosomes impact therapy for males with Fragile X Syndrome?
Even though males typically do not undergo X-chromosome inactivation like females, the concept of freeing inactivated X chromosomes could still enhance treatment outcomes for males with Fragile X Syndrome. This approach might restore function to specific genes affected by mutations, potentially alleviating the disorder’s effects.
What challenges remain in the research of X-chromosome inactivation?
Despite significant progress, challenges remain in fully understanding how X-chromosome inactivation works and why certain genes remain unaffected when therapies are applied. Further research is needed to clarify these mechanisms and enhance the therapeutic implications of restoring gene function in disorders like Fragile X Syndrome and Rett Syndrome.
Will the findings related to X-chromosome inactivation lead to clinical trials?
Yes, the research conducted, particularly in Jeannie Lee’s lab, is paving the way for clinical trials. The developed approaches for unsilencing X-linked genes in isolated cells show promise as potential treatments for genetic disorders, and ongoing safety studies aim to optimize these therapies before moving into clinical settings.
| Key Points | |
|---|---|
| Females have two X chromosomes, males have one. | Inactivation of one X chromosome is essential for gene balance in females. |
| X-inactivation is orchestrated by a gel-like substance surrounding chromosomes. | The gel allows for easier access and interaction with X-chromosome-specific proteins. |
| The RNA molecule Xist plays a crucial role in this inactivation process. | Xist alters the gel-like substance’s properties, enabling silencing of the X chromosome. |
| Restoring function to muted genes on one X chromosome could help treat disorders like Fragile X and Rett Syndromes. | Future treatments may allow unsilencing of the healthy gene copy for therapeutic benefits. |
| Research into X-chromosome inactivation has potential clinical applications and aims to begin trials soon. | The process offers hope for minimal side effects while targeting mutated genes. |
Summary
X-chromosome inactivation is a crucial biological process that ensures gene dosage balance between sexes. Females, with their two X chromosomes, undergo inactivation of one to avoid excess gene expression. Recent research from Jeannie T. Lee’s lab has uncovered the mechanisms behind this process, revealing the role of the Xist RNA molecule and a gel-like substance that facilitates silencing. This discovery opens up possibilities for treating genetic disorders caused by X-linked mutations, such as Fragile X Syndrome and Rett Syndrome. By potentially restoring the function of affected genes, this research marks a significant advancement in therapeutic strategies, promising hope for affected individuals.